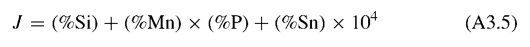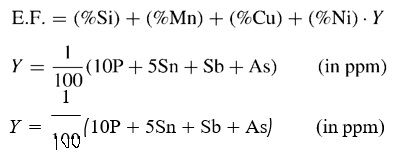Degradation of Materials in Service
- Meena Rezkallah, P.Eng.

- Jun 5, 2017
- 15 min read
A number of metallurgically based processes can occur in steels which contribute to loss of engineering strength, and even premature failure. Several of these are addressed in the following paragraphs.
Aging of Properties
A number of steels that have accumulated considerable service time are known to have experienced changes in their properties, usually to their detriment. This phenomenon has been called aging, and occurs in materials that are heat-treated or cold-worked to achieve high strength levels and to be used at elevated temperatures. These materials are potentially more susceptible to failure after the condition has developed.
‘‘Aging’’ in this case should not be confused with the same term used to represent the purposeful heat treatment performed to some types of nonferrous alloys. In the context being addressed here, aging refers to that normally very slowly progressing metallurgical reaction that occurs in a number of alloys while at operating temperatures for extended periods of time. Some specific types of this behavior (i.e., temper embrittlement and ‘‘885’’ embrittlement) are addressed in the paragraphs that follow.
Components that experience considerable service time contain materials that have aged with time. The materials of special interest are those that regularly experience higher operating temperatures; for example, ferritic steels above 900°F (482°C) and austenitic stainless steels at or above 1000°F (538°C). A study sponsored by the ASME Boiler and Pressure Vessel Code attempted to identify and quantify these effects. The effort was the result of concerns for near end-of-life seismic loadings in elevated-temperature nuclear boilers.
Data gathered from a number of sources have shown that the room and elevated temperature yield strengths of both ferritic and austenitic steels may degrade after long exposure times. Ultimate tensile strength is affected, but to a lesser degree. The yield strength reductions can amount to as much as 40 percent in ferritic and 20 percent in austenitic steels.
Creep tests have also been run after long-term (e.g., 10,000 h) static exposure to elevated temperatures. No substantial negative effect on creep properties were noted in these tests.
In the case of ASME Boiler and Pressure Vessel Code and ASME B31.1 Power Piping Code design and construction, the degradation of yield strength does not generally violate or invalidate the conservatism built into their design rules, as manifest by the allowable design stresses. For example, in Section 1 of the ASME Boiler Code, addressing design and construction of power boilers, the material design allowable stresses for wrought materials are established by applying the following factors to base material properties. The lowest calculated value of all the following is assumed as the design allowable stress at a given temperature:
¹⁄₄ specified minimum tensile strength at room temperature
²⁄₃ specified minimum yield strength at room temperature
¹⁄₄ tensile strength at the temperature of interest above room temperature
²⁄₃ yield strength at the temperature of interest above room temperature
67 percent of the average stress to cause rupture in 100,000 h
80 percent of the minimum stress to cause rupture in 100,000 h
100 percent of the stress to produce 0.01 percent strain in 1000 h
In this manner, short-term properties, stress rupture strength, and creep rate are all taken into account. Typically, at the lower end of the temperature use range, the factored tensile and yield strength controls, and at higher temperatures, the creep properties set the allowable stresses.
Since most aging occurs at the higher temperatures, tensile and yield strength degradation does not normally cause concern. However, if large shock loads can occur late in component life (as is possible under seismic conditions), these short- term, time-independent properties can be critical to the components’ continued safe operation.
As an illustration of the effect reduced yield strength has on fatigue, consider that with a lower yield strength, more plastic strain will result from a given high thermal, or mechanically induced stress. Since these stresses are usually due to operational transients, stress reversals can occur during continued operation (i.e., temperature stabilization at steady state, or ultimately, component shutdown). The greater the plastic strain cycle, the greater the damage, and the sooner the failure.
It is clear that as components experience increasing service time, they become less resilient to significant operational transients. That is, materials are less likely to withstand these transients than earlier in their life, not only because more cycles have continued to accumulate (toward an end-of-life limit), but also because material
strength properties are degrading with time.
Temper Embrittlement
Temper embrittlement is a phenomenon that occurs in carbon and alloy steels when aged in the temperature range roughly between 660 and 1020°F (350 and 550°C). The property most significantly affected is toughness. The time in which this occurs is a function of the steel’s chemical composition, heat treatment condition, fabrication history, and service temperature. The most severe degradation occurs in weld regions. Due to the extensive use of Cr–Mo steels in the petrochemical and power boiler industries, most of the studies have concentrated on this family of materials.
It has been recognized for some years that a steel’s susceptibility to temper embrittlement is due to the existence and amount of the trace elements antimony (Sb), phosphorous (P), tin (Sn), and arsenic (As), with P and Sn having the greatest effect. Other elements that may contribute to reduced toughness are silicon, manganese, and copper. Beneficial effects can be gained by additions of molydbenum and aluminum. The obvious dependency of temper embrittlement severity and chemistry has led to the development of a number of embrittlement factors.
Bruscato made the first attempt to combine the effects of various elements into a single factor, known as the embrittlement factor X, which is expressed as follows:
The concentration of elements is in parts per million (ppm). Miyano and Adachi arrived at a J-factor defined as:
Finally, Katsumata et al, asserted that the following embrittlement factor (E.F.) was appropriate for 2¹⁄₄ Cr–1Mo and 3 Cr–1Mo steels.
All of these are useful in assessing the relative susceptibility of various steel compositions to temper embrittlement. In all cases, the larger the factor, the more susceptible the particular heat of steel is to embrittlement.
The type of heat treatment applied to the materials may also affect a material’s susceptibility to temper embrittle. For example, a number of experimenters have confirmed that 2¹⁄₄ Cr–1 Mo alloy steels’ susceptibility increases as austenitizing temperature used during its heat treatment increases. Inversely, susceptibility is lowest after an intercritical hold, at a temperature between the lower and upper critical temperature (Ac1 and Ac3, respectively). This effect is believed to be associ- ated with the grain size achieved during the hold time, a larger grain being more detrimental. Although intercritically treated materials are less susceptible to temper embrittlement, they are weaker in the as-heat-treated condition.
In a parallel fashion, the degree of embrittlement, as measured by loss of toughness or shift of nil ductility temperature, is decreased if the material is more substantially tempered prior to the embrittling treatment. In this context, ‘‘tempered’’ represents the planned heat treatment that typically follows a normalizing or austenitizing and rapid quenching operation. A longer or higher temperature temper results in a softer, less strong, and more ductile condition, usually accompanied by good fracture toughness.
Luckily, the temper embrittled condition is reversible. Heat treatment for short periods of time at temperatures well above the upper critical will result in reestablish- ing nearly virgin properties in these materials.
Hydrogen Attack
Hydrogen attack is one of the most important problems with materials used in ammonia synthesis, oil refining, and coal gasification equipment.
The first major failure of an ammonia convertor attributable to hydrogen damage was in 1933. Since then more failures and untold damage to materials have accumulated, with the majority of damage occurring in the welds or weld heat-affected zones of these components.
When carbon and low-alloy steels are held in hydrogen at high temperature and pressure for an extended period of time, these materials can suffer degrading effects to their tensile and creep rupture properties. This is accompanied by the formation of intergranular fissures, blisters on the surface, and loss of carbon content (decarburization). The phenomenon is called hydrogen attack and is generally attributed to the formation of methane (CH4) within the steel.
The microstructural damage occurs when methane bubbles form and grow around precipitates at the grain boundaries within the material. The continued growth of the bubbles causes grains to separate along their boundaries and the bubbles, or voids, to coalesce. The rate of growth of the bubbles is a function of the ease by which the steel carbides give up carbon atoms to the intruding hydrogen atoms to form the methane. The more stable the carbide, the slower this reaction will take place. Thus, it has been long recognized that additions of chromium and molybdenum, both strong carbide stablizers, improves hydrogen-attack resistance of steels. Addition of other carbide-stabilizing elements such as titanium and tungsten has also assisted in reducing susceptibility.
Weld regions are more susceptible to hydrogen damage because they possess less stable carbides. It is also readily apparent that carbon content of the base material is an important variable in determining the susceptibility of a steel to hydrogen damage. In general, steels used in this service are kept below 0.20 weight percent carbon content. Certain other elements, such as nickel and copper, are known to also have a detrimental effect.
Nelson curves have proven indispensable in the selection of materials in hydrogen service. These curves (see Fig. A3.20. for an example) were originally based on experience gathered over several decades, and have been revised as new experience has been gained. These curves identify a ‘‘safe’’ regime in which an alloy will perform acceptably at various temperatures and hydrogen partial pressures. Where these curves have proven unconservative have been associated with weld heat- affected zones that had been inadequately postweld heat-treated (PWHT). The high residual stresses and high hardness left in the weld region contribute to accelerated damage. For this reason, most specifications for hydrogen service equipment stipu- late a maximum hardness in weld regions that will assure adequacy of the PWHT. The limit is usually placed at 210 Brinell hardness, corresponding approximately to a 100,000 psi (690 Ma) ultimate tensile strength.
Austenitic stainless steels are essentially immune to hydrogen damage. The numerous sites within the FCC lattice in which the hydrogen atoms can be safely accommodated, and the inherent ductility of the lattice, gives austenitic materials this freedom from hydrogen damage. However, when stainless overlay weld metal has been used over carbon or low-alloy vessel steels, hydrogen-induced cracking can occur at the weld fusion line just inside the ferritic material.
‘‘885°F’’ (474°C) Embrittlement
One of the limitations of ferritic stainless steels (those alloys of iron possessing greater than about 14 pecent chromium) has been the loss of toughness at room temperature that occurs after these materials are exposed for long times to tempera- tures in the range of 610 to 1000°F (320 to 538°C). This is commonly referred to as 885°F (474°C) embrittlement, corresponding approximately to the temperature at which many of the alloys degrade the fastest.
The compositional effects in commercial alloys on 885°F (474°C) embrittlement have not been systematically investigated. However, it is clear that the degree of embrittlement increases as chromium content increases. The effects that other elements may have is not clear. Of these, most important is carbon, and it has been reported as having from no effect to a retarding effect on embrittlement.
This phenomenon results in increased hardness and strength, with a correspond- ing decrease in ductility, fracture toughness, and a decrease in corrosion resistance. Loss of toughness can be particularly severe, and in fact has tended to relegate the use of this class of alloy to temperature regimes below which significant embrittlement can occur.
Graphitization
Graphitization is a time- and temperature-dependent nucleation and growth process, in which iron carbide in the form of pearlite first spheroidizes, and later forms graphite nodules. There are two general types:
Formation of randomly, relatively uniformly distributed graphite nodules in the steel. This reduces the room temperature mechanical strength somewhat, but does not affect the creep-rupture strength at elevated temperature.
A concentrated formation of graphite most frequently along the edges of the heat-affected zone of weldments. This is referred to as chain graphite, since a plane of nodules exists paralleling the weld bead contours.
The formation of these nodules, when aligned through the wall of a pressure part, creates planes of weakness, subject to rupture. Fracture characteristically occurs without prior warning.
The first graphitization failure of a low-carbon steam piping material occurred in the early 1940s. The failure occurred after five and a half years of service in a steam line made of aluminum-killed carbon-molydenum steel. The fracture surface was located approximately ¹⁄₁₆ in (1.6 mm) from the fusion zone of a butt weld. The failure precipitated numerous and extensive research programs to understand the key variables of the mechanism and to determine the steels which would resist graphitization.
Research has helped in the understanding of the problem, and led to restrictions adopted by the various design codes on use of materials subject to graphitization. Carbon steel and carbon-molybdenum grades are the most susceptible to this degradation process, with the latter being more so. Relative susceptibility of these two grades is also dependent on the steel’s aluminum content; the more aluminum, the greater the susceptibility. Additions of chromium in amounts as low as 0.5 weight percent make the steel essentially immune to graphitization.
The ASME Code permits the use of carbon and carbon-molybdenum steels in ASME Section 1 boiler applications up to 1000°F (538°C). A cautionary note is provided in the allowable stress tables of Section I indicating the carbon steels and carbon-molybdenum steels may be susceptible to graphitization at temperatures above about 800 and 875°F (427 to 468°C), respectively. ASME B31.1 has a similar precautionary note specifying limits of 775 and 850°F (413 and 454°C), respectively. Graphitization is a mechanism dependent on diffusion and is not associated with a precise temperature of initiation (it occurs sooner at higher temperatures). Thus, the differences between the design codes only reflect different levels of conservatism in dealing with the failure mode. Many manufacturers extend even more severe restrictions, some prohibiting the use of these steels in piping applications outside the boiler or pressure vessel where rupture creates a serious safety hazard. Substitution of chromium-containing steel grades, such as SA.335 P2(¹⁄₂Cr-¹⁄₂Mo), P11 (1¹⁄₄Cr-¹⁄₂Mo), and P22 (2¹⁄₄Cr-1Mo), is normally recommended for these applica- tions. Grade P91 (9Cr-1Mo-V) is increasingly being used in high-temperature appli- cations where use of P11 and P22 is not desirable due to their reduced mechanical strength.
Intergranular Attack
When an unstabilized austenitic stainless steel is held at a temperature within the range of 850 to 1500°F (454 to 816°C), chromium carbides will quickly and preferentially form at the austenitic grain boundaries. The formation of these car- bides deletes the surrounding grain matrix of chromium atoms, rendering the thin zone adjacent to grain boundary susceptible to corrosive attack in aqueous environments. This condition is called sensitization, and the resulting corrosion is termed intergranular attack (IGA). When also in the presence of local high-tension stresses, the result can be intergranular stress corrosion cracking (IGSCC). Avoidance of these failure mechanisms is best achieved by minimizing sensitization (fast cool from anneal; stabilized or L-grade steels), and eliminating local stresses.
The area of piping components most often attacked is weld regions. Sensitization can readily occur in a narrow band of base material in the heat-affected zone, caused by the heat of the weld pool. Corrosion of this area has been called knife line attack due to the characteristic appearance of a thin crack along a weld edge.
Sigmatization
A hard, brittle, nonmagnetic phase will form in some Fe-Cr and Fe-Ni-Cr alloys upon prolonged exposure to temperatures between about 1100 and 1475°F (593 and 800°C). Those austentic stainless steels containing higher alloy content, such as type 310 (25% Cr–20% Ni) are susceptible, as well as any grades that possess residual ferrite in their microstructure, a constituent which will transform to sigma, preferentially at grain boundaries.
The most deterimental effect of sigma is reduction of toughness. Charpy V-notch impact toughness can degrade to less than 10 ft · lb (14 joules) at room temperature if as much as 10 percent of the volume of material transforms. Toughness is usually not significantly degraded at higher temperatures, above about 1000°F (538°C).
Chemically, sigma is not as resistant to oxidizing media as the austenite, such as acidic environments, thus, the materials will undergo intergranular attack.
At normal metal operating temperatures in power plants, sigmatization of pres- sure piping made of these high-alloy materials takes very long times to form. Once formed, the phase can be redissolved by subjecting the material to an annealing heat treatment.
Creep Damage and Estimation of Remaining Creep Life
The type of damage observed in components operating at high temperatures, and high stress, typically progresses in stages occurring over a considerable period of time. Elongation or swelling of the component may be observed. Material damage manifests itself in the microstructure in characteristic form at grain boundaries. Voids will form first, which then subsequently link up to form cracks. These cracks increase in size or severity as the end-of-life condition is approached. Severe-damage indications invariably signal the need for near-term corrective action. Such corrective action may entail repair or replacement of the component in question, depending upon the extent of the damage and the feasibility of repair. It is important to note that, except in the most severe cases, damage is not readily detectable by the naked eye, or even by conventional nondestructive techniques such as ultrasonic, magnetic particle, or liquid penetrant examination methods.
The degree of microstructural damage can be assessed by conventional metallographic procedures that may either take a destructive sampling approach or use nondestructive in-place (in-situ) methods. Since the determination of the structural damage allows for a ready estimation of expended creep-rupture life, these inspection methods have recently been adopted to piping and other structural components. The power piping industry, in particular, has seen a wholesale application of metallographic examination to components that have experienced extensive time in elevated temperature service. Several serious steam line ruptures have caused deaths, serious injury, and significant lost operating time at fossil energy power plants. The steam lines that have come under the greatest scrutiny are reheat super heater piping which, based on their relatively large diameters and thin walls, had been made from rolled and welded plate. The failures have been associated with the longitudinal weld regions, which are inherently more susceptible to problems due to danger of latent defects (lack of fusion, slag entrapment, solidification cracks), and the variabil- ity in mechanical properties across the welds heat-affected zone.Destructive sampling of material surfaces of suspected creep-damaged components, to allow for metallographic examination, has evolved to the point where there can be minimal disturbance to surrounding material. Test samples are either trepanned through thickness or smaller silver (boat-shaped) samples are removed by sawing, electro discharge machining, or other methods. However, arc gouging or any other form of heat-producing mechanism must be avoided. It not only can significantly metallurgically alter surrounding material but also can damage the destructive sample, sometimes rendering it unusable for microscopic analysis. The small samples, once properly removed, are metallographically prepared in the standard fashion. These are then examined at high magnification in metallurgical micro- scopes for evidence of creep damage. The area from which this sample was removed must be weld repaired, employing the required preheat, postweld heat treatment, and weld inspections.
Alternately, an evaluation of microstructure can be performed in place on the component surface, in the area of interest using a procedure called replication, which provides, in a manner of speaking, a fingerprint image of the surface. The area to be examined is first carefully polished to a mirrorlike finish using ever- increasing fineness of sandpapers or grinding disks, and then polishing compounds. The surface is then etched with an appropriate acid. A thin, softened plastic film is then applied to the surface. Upon drying, the film hardens, retaining the micro- structure in relief. When properly done by skilled technicians, the resolution of the metal structure at magnifications up to 500X or higher is almost equal to that achieved on an actual metal sample. The disadvantage of the replication method is that only the surface of the material can be examined, leaving any subsurface damage undetected. However, this method has proven useful when applied to weld regions, or other high-stressed areas where damage is suspected.
Remaining creep-life determination done in this fashion is not exact; the correla- tion between the type and degree of damage, and expended creep life is only approximate. In most cases, follow-up inspection several years hence is necessary to determine the rate of damage progression. Usually, when a network of micro- cracks has been generated, it is time to consider repair or replacement.
The science of estimating the expected growth rate of these cracks by creep evolved very rapidly in the 1980s. Armed with sufficient baseline creep data of a given alloy, formulas have been developed that can predict creep crack growth rates reasonably accurately. Analysis can also be made whether a pipeline would ‘‘leak before break’’; that is, weep fluid for a time prior to catastrophic rupture. All of these tools are available to the piping designer and to operating management.
Oxide Thickness and Estimation of Remaining Creep Life
Another method for estimating remaining creep life of certain high-temperature tubing and piping components considers the amount of metal oxide scale that has formed on the metals surface. Understandably, this method only applies when the tubular items contain relatively benign substances under oxidizing conditions. It has found its use in steam-carrying piping and components. This method is based on the knowledge that a given thickness of oxide scale on the tube or pipe surface represents growth for a certain time at some temperature. Since oxide growth kinetics of many alloys are well characterized, the effective temperature at which the tube was operating for a known time (service life) can be estimated. The combination of effective temperature and time can then be compared to the typical creep life of the alloy at an applied stress or stresses that are known to have acted on the component during its service life.
As noted, the two principal tools needed by the metallurgist to estimate life using the oxide measurement technique are (1) steam oxidation data for the alloy in question, and (2) uniaxial creep-rupture data for that alloy across the temperature range of interest. This latter information can be found for many of the most widely used ferrous alloy piping materials in ASTM references. The specific steps followed in this approach are as follows:
Oxide thickness is measured either metallographically on a sample or using specialized ultrasonic techniques. Operating time is known.
The effective operating temperature is determined from the oxidation data. The effective temperature is defined as the constant temperature that the particular tube metal would have had to have operated at for the known service time to have resulted in the measured oxide thickness. (This is an approximation, since the tube or pipe would have operated at various temperatures, perhaps even in upset conditions well above the design temperature limit.)
The hoop stress is calculated using an appropriate formula, knowing the tube or pipe size and operating pressure.
The Larsen-Miller Parameter (LMP) is calculated for the service time and effec- tive temperature of the subject tube. The LMP is defined as:
Uniaxial creep-rupture data is obtained for the alloy in question. Examples of data for 1¹⁄₄Cr-¹⁄₂Mo-Si and 2¹⁄₄Cr-1Mo, taken from creep data sources ASTM DS50 and DS652 are shown as Fig. A3.21 and Fig. A3.22. This rupture data is normally represented by curves of minimum and average behavior, and lists applied stress versus LMP.
The ASTM rupture curve is entered on the stress axis at the level of appropriate calculated operating stress (from step 3). In this manner, the LMP representing the expected minimum and average total creep life at that stress is determined.
The operating LMP calculated in step 4 is compared to the LMPs derived in step 6. The differential in time represented by these parameters can be easily calculated from the Larsen-Miller formula, and the percentage of expended life versus minimum and average expected life can be determined by taking a ratio of these values.
This method for estimating remaining creep life has found its greatest use in the fossil power boiler industry, particularly for ferritic alloy steam piping and super heater tubing. Since a great majority of the operating power boilers in the United States are approaching their originally intended lifetime, the method is critical for establishing when major repair or replacement is necessary to restore the unit to safer and more reliable operation. #Little_PEng